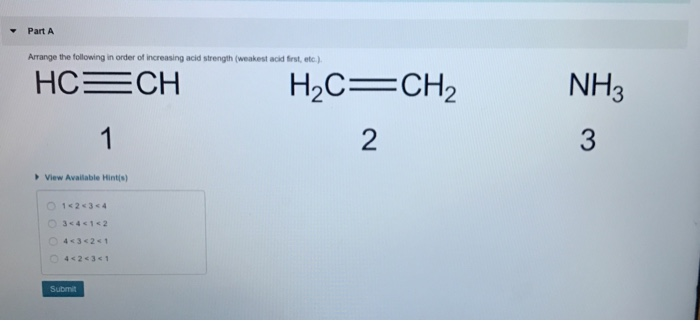
Arrange the Following in Order of Increasing Acid Strength
A 032M solution of phenol which is a weak monoprotic acid has app of 522. Arrange the following acids in order of greatest at the top of the list to least at the bottom of the list amount of H3O produced in solution.

Arrange The Following In Increasing Order Of Their Acidic Strength
Place the following acids in order from smallest to largest Ka value.

. HClO3 HIO3 HBrO3. So the acidic strength increases as follow. HClO3 HBrO3 and HIO3.
- Thus the acids can be arranged in the order of increasing acid strength as follows HOItext. The correct order of acidic strength of the following is I Oxalic acid. Add your answer and earn points.
Arrange the following in decreasing order of acid strength. HClO3 HBrO3 and HIO3. Thus the acid strength order is HF HCI HBr HI.
It should be noted that due to the high electronegativity and very small size of fluorine it can form only one oxoacid HOF whereas the other elements of the halogen family can form several oxoacids. HIO HBrO HClO HClO 2 HClO 3. Acetic acid is ten times weaker an acid than formic acid first two entries in the second row confirming the electron donating character of an alkyl group relative to.
Since the bond dissociation enthalpy of H X bond decreases from H F to H-l as the size of atom increases from F to I. Arrange in order of increasing acidic strength. Based on molecular structure arrange the following sets of oxyacids in order of increasing acid strength.
Asked Aug 17 2019 in Chemistry by Yotun. Arrange the following in increasing order of acidic strength. FCH2COOH is the strongest acid out of all.
Also among HClO HBrO and HIO as the electronegativity of the halogen increases the acidic strength increases. Solution for Assuming 01 M concentrations arrange the following acid solutions in order of increasing pH. Benzoic acid HC7H5O2 Ka 63 105 chlorous.
However due to ortho-effect o -nitro-benzoic add is the stronger add then p -nitrobenzoic add. CH3OH CH3CH2OH CH3CHOHCH3 CH33COH - Chemistry Advertisement Remove all ads. HIO3 HBrO3 HClO3.
HIO HBrO HClO. Arrange the following in order of increasing acid strength i HBr HCl HF HIii CH4 H2O HF NH3. Asked Mar 6 2019 in Chemistry by Paleolita.
Which of the following is the strongest acid CH3COOH brch2cooh ClCH2COOH FCH2COOH. Acid strength depends on strength of H X bond weaker the bond easily to give H. According to this among given compounds the acidic strength increases as follow.
Electron-donating substituents tend to decrease while electron-withdrawing substituents tend to increase the acidic strength of substituted benzoic acids relative to benzoic adds. Hliles6259 is waiting for your help. C H 32C H C OOH I C H 3C H 2C H BrC OOH I I C H 3C H BrC H 2C OOH I I I C H 3 2 C H C O O H I C H 3 C H 2 C H B r C O O H I I C H 3 C H B r C H 2 C O O H I I I.
-Part A Arrange the following in order of increasing acid strength weakest acld first etc. Based on molecular structure arrange the binary compounds in order of increasing acid strength. Place the following acids in order from smallest to largest Ka value.
Place all the steps required to calculate the value of Ka for phenol in the correct order. HOOC-COOH II Malonic acid HOOC-CH_2-COOH III Succinic acid HOOC-CH_2_2-COOH IV Glutaric acid HOOC-CH_2_3-COOH. Arrange the following acids in their increasing order of acidic strength.
Ka 603 10-62 032 112 10-10. How do you arrange in increasing order of acidic strength. See what the community says and unlock a badge.
Arrange the following in the increasing order of their property indicated any 2A Benzoic acid Phenol Picric acid Salicylic acid pka valuesB Acetaldehyde Acetone Methyl tert butyl ketone reactivity towards NH2OHC ethanol ethanoic acid benzoic acid boiling pointAnswerA Picric. Acetic acid chloro acetic acid propanoic acid formic acid. H F H C I H B r H I.
Previous question Next question. Arrange the following compounds in order of increasing acid strength weakest at the top to strongest at the bottom of the list. HClO HClO 2 HClO 3.
Assume that phenol 032. Arrange the following carboxylic acids in increasing order of their acid strength. H2te hi h2s nah - 9374563.
Acid strength of HF HCI HBr and HI depends upon their bond dissociation enthalpies. Based on molecular structure arrange the following sets of oxyacids in order of increasing acid strength. Arrange the acids HOBr HBrO3 and HBrO2 in order of increasing acid strength.
NH3 H2C CH2 2 HC-CH 3 View Available Hint s 14234 34412 4342s 1 42431 Submit. Since bond dissociation enthalpy dcreases from H F to H I as the size of atom increases from F to I Hence the correct increasing order of acid strength is. Ionization 603 10-6 032 100.
100 23 ratings Transcribed image text.
Solved Arrange The Following In Order Of Increasing Acid Chegg Com

Arrange The Following Compounds In Increasing Order Of Their Acidic Strengt Youtube
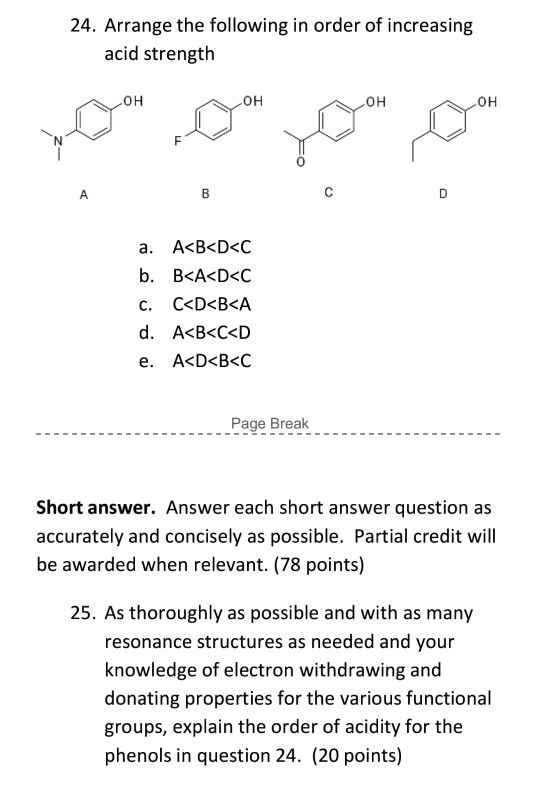
Solved 24 Arrange The Following In Order Of Increasing Acid Chegg Com
Comments
Post a Comment